
Breath Actuated Inhaler


Dry Powder Inhaler

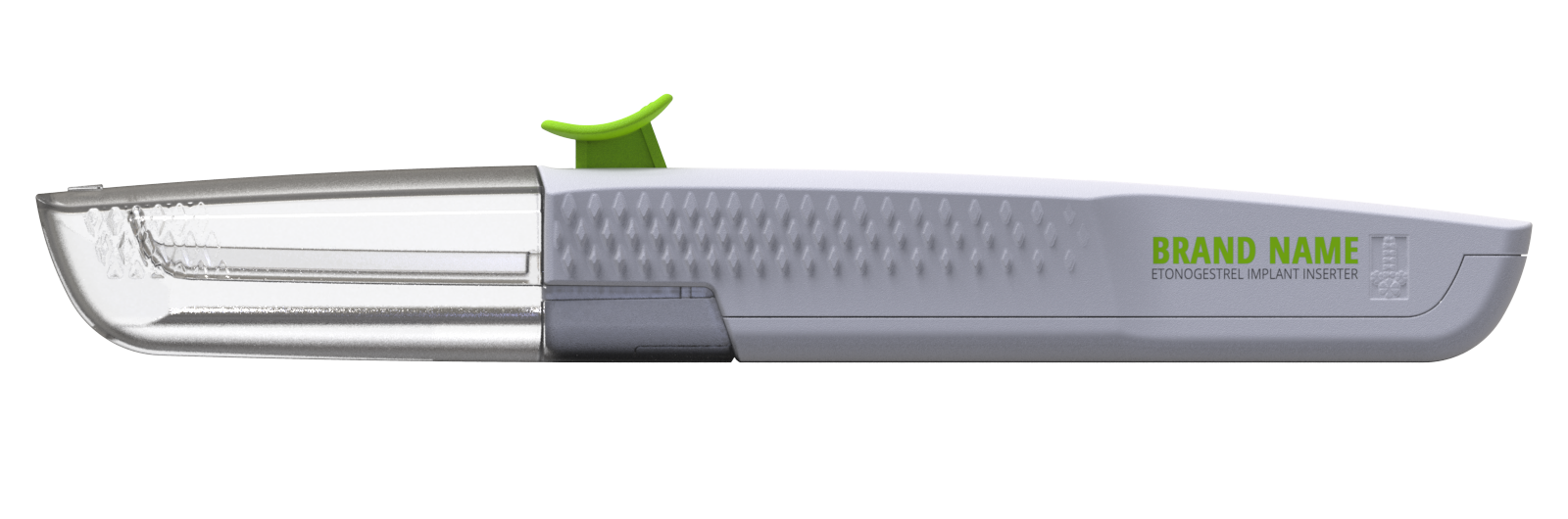

Lupin Pharma brought in Insight to help develop a generic breath-actuated inhaler, aiming to work around existing IP and fast-track an FDA ANDA submission. The project’s success led to an ongoing partnership, with Lupin returning for more development work, including a dry powder inhaler, a birth control inserter, and other ongoing projects.





